Current criteria to swab for COVID-19 in the UK:
Clinical : Pneumonia clinical or radiological, ARDS, Flu like illness (fever, cough whether productive or non productive, sore throat, runny nose, myalgia. Less people present initially with diarrhea 10% and they had the worst prognosis later from the Wuhan experience)
Or Epidemiological : last 14 days in tier 1 or tier 2 countries. Or contact with confirmed case of COVID-19.
-Incubation period 4 - 14 days. People are infective mostly in the first 5 days when the symptoms are milder. Deterioration happens after day 10 of the symptoms (when the body enter into the adaptive immunity stage)
-PCR for COVID19 sensitive for 70% only, may be initially negative, a repeat PCR in high suspected cases recommended in 48 -72 hours.
-CT Thorax is sensitive for 97% of cases and could help in diagnosis of patient with initial negative PCR (positive CT Thorax for COVID-19 is patchy ground-glass appearance in the lung peripheries, it does not cause cavitation or lymphadenopathy)
- Old age >70, High SOFA score, and d-dimers >1000 are risk factors for poor prognosis.
Pediatrics guidelines if needed please contact me freely and i will provide you details with evidence.
-Lymphopenia with normal WBC count is common in 80%, mild thrombocytopenia , high CRP and ferritin tract with disease severity.
-High tropronin found in half of the dead patients (perhaps as IHD was a common risk factor, likely it is troponin leakage form sepsis /strain? some reported cases for severe myocarditis). Considered as a marker for poor prognosis.
-Give empirical antibiotics in the golden hour as per the normal sepsis protocol . Blood cultures./Procalcitonin. Review ABX in 48 hours.
-NO IV Fluids, unless there is an evidence of hypo-perfusion.
-No role of steroids as it delays viral clearance, unless the patient has obstructive airway disease or Septic shock not fluid or vasopressor responsive
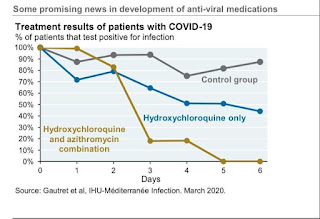
-Controversial role of Hydroxychloroquine: it interfers with cellular ACE receptors in the lung, hence has some anti-viral activities.
-Some Antiviral therapies like Remdesivir, combination of Lopinavir/Ritonavir/Ribavirin is of benefit in MERS, no evidence of use it in COVID-19, Clinical trials are taking place currently. If the patient deteriorated, consider them after discussion with the local ID team. No role of using Oseltamavir in COVID-19 unless influenza is considered when you admit the patient.
- Cytokines storm inhibitors in COVID-19: as the disease cause a cytokines storm (elevated inflammatory markers like CRP, ESR, ferritin), selective inhibition of cytokines Tocilizumab showed some benefit in phase 3 RCT in China. The drug was eventually licensed in China for severe COVID-19 infection.
- COVID-19 may cause secondary HLH?
-Severe respiratory failure/?ARDS: ITU for early Intubation and ventilation with low TV and allow for permissive hypercapenia, prone, paralysis, APRV, ECMO.
(HFNO and NIV is not recommended)
-Cardiac arrest in COVID-19:
Identify resuscitation status for any ?COVID-19 hospital admission.
Recognize cardiac arrest by absence signs of life and absent carotid pulse. Do not do listen and feel for breathing.
Wear full PPE (FFP3, Visor, Gloves, plastic apron) then start CPR. The likely cause is a hypoxic arrest. However, the recommendation from resuscitation council UK is to start with CRP. Early ventilation is advised.
Clinical : Pneumonia clinical or radiological, ARDS, Flu like illness (fever, cough whether productive or non productive, sore throat, runny nose, myalgia. Less people present initially with diarrhea 10% and they had the worst prognosis later from the Wuhan experience)
Or Epidemiological : last 14 days in tier 1 or tier 2 countries. Or contact with confirmed case of COVID-19.
-Incubation period 4 - 14 days. People are infective mostly in the first 5 days when the symptoms are milder. Deterioration happens after day 10 of the symptoms (when the body enter into the adaptive immunity stage)
-PCR for COVID19 sensitive for 70% only, may be initially negative, a repeat PCR in high suspected cases recommended in 48 -72 hours.
-CT Thorax is sensitive for 97% of cases and could help in diagnosis of patient with initial negative PCR (positive CT Thorax for COVID-19 is patchy ground-glass appearance in the lung peripheries, it does not cause cavitation or lymphadenopathy)
- Old age >70, High SOFA score, and d-dimers >1000 are risk factors for poor prognosis.
Pediatrics guidelines if needed please contact me freely and i will provide you details with evidence.
-Lymphopenia with normal WBC count is common in 80%, mild thrombocytopenia , high CRP and ferritin tract with disease severity.
-High tropronin found in half of the dead patients (perhaps as IHD was a common risk factor, likely it is troponin leakage form sepsis /strain? some reported cases for severe myocarditis). Considered as a marker for poor prognosis.
-Give empirical antibiotics in the golden hour as per the normal sepsis protocol . Blood cultures./Procalcitonin. Review ABX in 48 hours.
-NO IV Fluids, unless there is an evidence of hypo-perfusion.
-No role of steroids as it delays viral clearance, unless the patient has obstructive airway disease or Septic shock not fluid or vasopressor responsive
-Controversial role of Hydroxychloroquine: it interfers with cellular ACE receptors in the lung, hence has some anti-viral activities.
-Some Antiviral therapies like Remdesivir, combination of Lopinavir/Ritonavir/Ribavirin is of benefit in MERS, no evidence of use it in COVID-19, Clinical trials are taking place currently. If the patient deteriorated, consider them after discussion with the local ID team. No role of using Oseltamavir in COVID-19 unless influenza is considered when you admit the patient.
- Cytokines storm inhibitors in COVID-19: as the disease cause a cytokines storm (elevated inflammatory markers like CRP, ESR, ferritin), selective inhibition of cytokines Tocilizumab showed some benefit in phase 3 RCT in China. The drug was eventually licensed in China for severe COVID-19 infection.
- COVID-19 may cause secondary HLH?
-Severe respiratory failure/?ARDS: ITU for early Intubation and ventilation with low TV and allow for permissive hypercapenia, prone, paralysis, APRV, ECMO.
(HFNO and NIV is not recommended)
-Cardiac arrest in COVID-19:
Identify resuscitation status for any ?COVID-19 hospital admission.
Recognize cardiac arrest by absence signs of life and absent carotid pulse. Do not do listen and feel for breathing.
Wear full PPE (FFP3, Visor, Gloves, plastic apron) then start CPR. The likely cause is a hypoxic arrest. However, the recommendation from resuscitation council UK is to start with CRP. Early ventilation is advised.

No comments:
Post a Comment